Explore the fascinating world of liquid layers, how they form, interact, and shape everything from science to everyday life. Learn how density, temperature, and other factors influence liquid layering.
What Are Liquid Layers?
Liquid layers are exactly what they sound like—distinct, stacked levels of different liquids, usually separated by their varying physical properties like density, temperature, or salinity. You may have seen this happen in your own kitchen when oil floats on water or in a fancy layered cocktail. But this phenomenon isn’t just fun for bartenders or home experiments; it plays a vital role in natural systems, industrial processes, and scientific research.
The concept of liquid layers is grounded in physics and chemistry. When two or more liquids come into contact and don’t mix, they naturally form layers. This occurs due to differences in molecular composition, density, and surface tension. Denser liquids sink, while less dense liquids rise. These layers can remain stable or shift depending on temperature changes, vibrations, or external agitation. This layering is crucial in various contexts, from oceanic thermoclines to chemical labs.
The Science Behind Liquid Layers
At the heart of liquid layering lies the principle of density. Density is defined as mass per unit volume, and it dictates how substances interact when combined. When two immiscible liquids—that is, liquids that do not mix—are poured into the same container, the denser one will settle at the bottom.
Think about oil and water. Oil has a lower density than water, so it naturally floats on top. The boundary between them is visible and remains stable unless stirred or mixed. The fascinating part is that this isn’t just a random occurrence; it is a predictable outcome based on well-understood scientific principles.
Another factor influencing liquid layers is temperature. Warmer liquids are generally less dense than colder ones. This temperature-driven density difference often leads to layering in natural bodies of water. For instance, lakes in summer exhibit clear thermal layers that separate warmer surface water from colder, denser bottom water.
Real-Life Examples of Liquid Layers
Liquid layers aren’t just academic or theoretical—they’re everywhere. One of the most relatable examples is in cooking. Salad dressings often separate into layers of oil and vinegar. Without emulsifiers or shaking, these liquids will sit in distinct layers indefinitely.
Another compelling example is in geology and hydrology. Aquifers often have multiple layers of water and minerals. Similarly, oil reservoirs feature stratified liquid layers, which drilling experts study meticulously before extraction. The ocean is perhaps the most dramatic example. The stratification of ocean layers based on salinity and temperature is critical to marine life and global climate patterns.
In the lab, scientists often use layered liquids for separation techniques like liquid-liquid extraction. The precise control of layers allows chemists to isolate specific compounds from mixtures, a practice widely used in pharmaceuticals and chemical engineering.
How Density Influences Layering

Density plays a starring role in determining how liquid layers form. When two liquids of different densities come into contact, they do not mix evenly unless forced to. The denser liquid will settle at the bottom while the less dense liquid rises to the top.
This phenomenon can be demonstrated with a simple experiment. Fill a glass with honey, followed by dish soap, then water, and finally vegetable oil. You will notice distinct layers forming, each sitting atop the next based on density. This basic principle is leveraged in industries ranging from oil refining to food production.
The importance of density in the natural world cannot be overstated. It governs not only water bodies but also atmospheric conditions. Even in weather systems, different air masses with varying humidity and temperature can form layers that affect climate and precipitation patterns.
Temperature and Its Role in Liquid Layers
Temperature is another critical factor in the formation and stability of liquid layers. When a liquid is heated, it expands, causing its density to decrease. Conversely, when it cools, it contracts and becomes denser. This principle is evident in natural water bodies like lakes and oceans.
During summer, lakes often develop a thermocline—a layer where water temperature changes rapidly with depth. The top layer is warm and less dense, while the bottom layer is cooler and denser. This thermal stratification influences aquatic life, nutrient distribution, and even recreational water use.
In industrial processes, temperature control is essential for maintaining desired liquid layers. For instance, in chemical processing, maintaining temperature gradients ensures effective separation of compounds. Improper temperature control can disrupt these layers, leading to inefficiencies and potential hazards.
Surface Tension and Immiscibility
Surface tension also contributes to the behavior of liquid layers. Surface tension is the cohesive force at the surface of a liquid that makes it behave like an elastic sheet. It plays a subtle but significant role in keeping layers from mixing too easily.
For instance, when oil and water are in a container, the surface tension of water is higher than that of oil. This difference enhances the immiscibility of the two liquids, helping them form distinct layers. Surface tension also affects how droplets form and move within layered liquids, which is important in areas like inkjet printing and pharmaceuticals.
Immiscibility, the inability of liquids to mix, is foundational to liquid layering. It is determined by the molecular structure of the liquids involved. Polar and non-polar substances, like water and oil respectively, do not mix because their molecules interact differently. This immiscibility is what keeps the layers intact.
Liquid Layers in Oceans and Lakes
The world’s oceans and lakes are some of the most complex and significant examples of liquid layers. These bodies of water are not uniform but instead have structured layers based on temperature, salinity, and density. Understanding these layers is vital for climate science, marine biology, and navigation.
In oceans, three main layers typically exist: the surface mixed layer, the thermocline, and the deep ocean layer. The surface layer is where sunlight penetrates, enabling photosynthesis. The thermocline is where temperature changes sharply with depth, and the deep layer is cold and dense. Each layer supports different kinds of marine life and has distinct chemical properties.
Lakes also exhibit seasonal layering. In summer, the top water is warm and less dense, while in winter, the bottom layers can be warmer than the icy surface. These patterns affect oxygen levels, nutrient cycles, and the overall health of aquatic ecosystems.
Industrial Applications of Liquid Layers
Liquid layers play a crucial role in various industries. In oil refining, the process of fractional distillation separates crude oil into different components based on boiling point and density, naturally forming layers of different hydrocarbons. Managing these layers efficiently is essential for product quality and safety.
In the food industry, emulsions like mayonnaise or salad dressings require a deep understanding of how to mix or layer liquids appropriately. Improper handling can lead to separation, spoilage, or unappetizing textures. Similarly, in the pharmaceutical industry, liquid layering helps in controlled drug release and precise chemical reactions.
In wastewater treatment, layered liquid systems help in separating contaminants from water. By understanding how different substances layer and react, engineers can design more efficient filtration and purification systems. This technology is especially important as global demand for clean water continues to rise.
How to Create and Observe Liquid Layers at Home
Creating liquid layers at home is both fun and educational. It’s a great way to visualize complex scientific principles in a hands-on way. All you need are liquids of different densities, like honey, dish soap, milk, water, oil, and rubbing alcohol.
Start by pouring the densest liquid into a transparent container. Slowly add each successive liquid down the side of the glass or using a spoon to reduce mixing. If done carefully, you’ll see distinct, colorful layers. This experiment is not just eye-catching but also a fantastic teaching tool for children and adults alike.
Add food coloring to each liquid to enhance the visual contrast. Observe how the layers remain separate and consider how changes in temperature or agitation could affect them. This kind of simple experiment lays the foundation for deeper scientific inquiry.
Common Misconceptions About Liquid Layers
One common misconception is that all liquids will layer if poured together. In reality, many liquids are miscible, meaning they mix completely when combined. For example, alcohol and water mix thoroughly because their molecules are compatible.
Another myth is that the order in which you pour the liquids doesn’t matter. While the final layering depends on density, the pouring technique can disrupt layers if done carelessly. It’s essential to pour slowly and gently, especially when adding a lighter liquid on top of a heavier one.
Some also believe that once layered, liquids will remain that way indefinitely. In truth, time, temperature changes, and movement can cause layers to blend. Understanding the factors that affect stability is crucial for both scientific accuracy and practical application.
The Role of Liquid Layers in Climate and Ecology
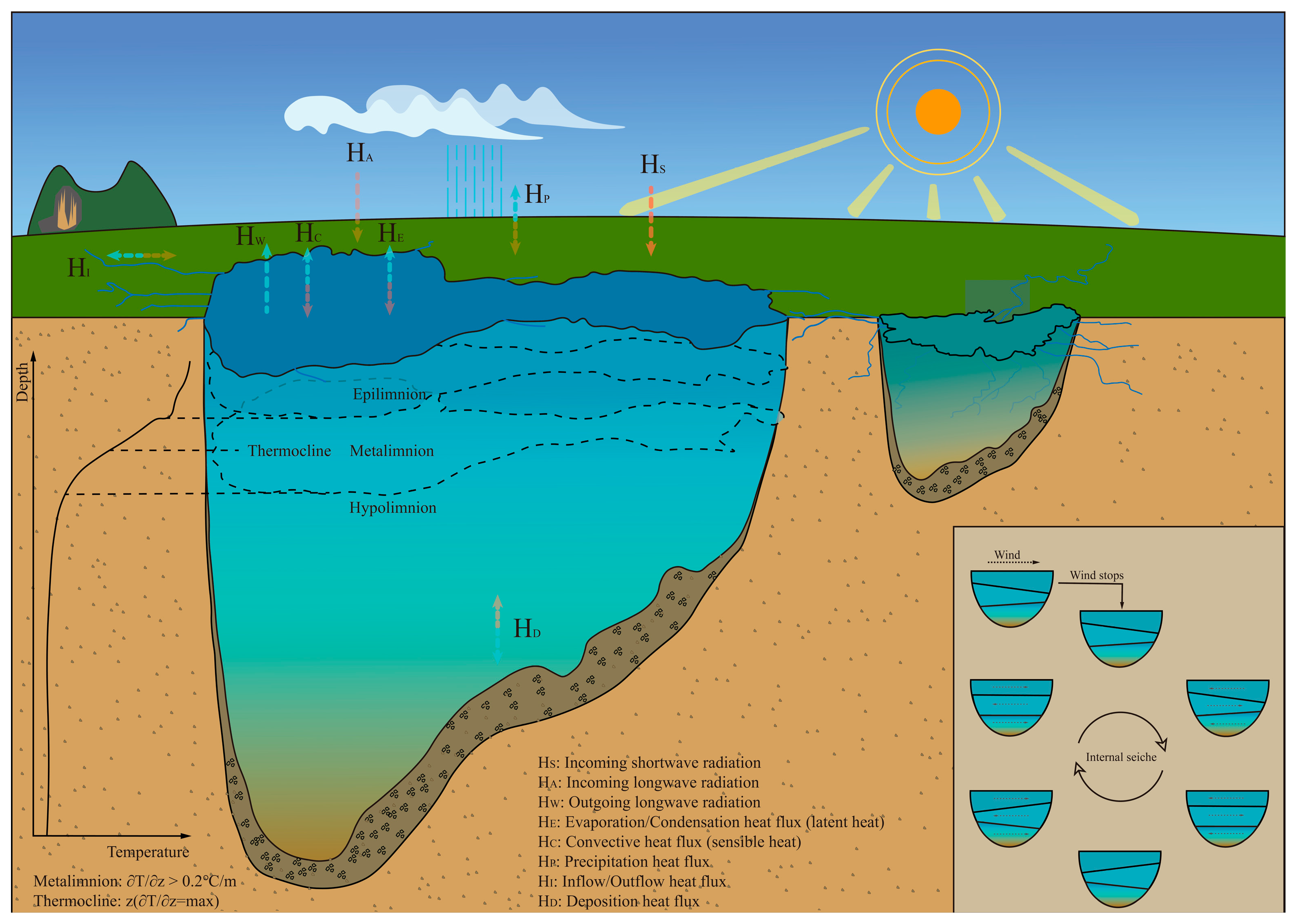
Liquid layers influence more than just scientific experiments and industrial processes; they have a profound impact on global climate and ecology. Oceanic layers regulate heat distribution, carbon storage, and marine biodiversity. Disruptions in these layers, such as through global warming, can lead to coral bleaching, fish migration, and altered weather patterns.
Similarly, in freshwater ecosystems, stratification affects oxygen availability and nutrient cycling. When lakes mix during seasonal turnovers, nutrients from the bottom rise to the surface, supporting plankton growth and sustaining the food web. Without these natural cycles, aquatic life would struggle to survive.
Understanding liquid layers is also essential for climate models. Scientists study how layered liquids behave to predict future environmental changes. These insights are used to design mitigation strategies, from marine conservation to atmospheric monitoring.
A Table of Common Liquids and Their Densities
| Liquid | Approx. Density (g/cm³) |
|---|---|
| Honey | 1.42 |
| Dish Soap | 1.06 |
| Water | 1.00 |
| Milk | 1.03 |
| Vegetable Oil | 0.92 |
| Rubbing Alcohol | 0.79 |
| Lamp Oil | 0.80 |
This table helps visualize why certain liquids layer the way they do. The higher the number, the denser the liquid.
Quotes About the Importance of Liquid Layers
“In every drop of water, there is a story of layered complexity waiting to be unraveled.”
“The layers in our oceans are like pages in a book, each telling a unique story of life, chemistry, and climate.”
Frequently Asked Questions
What are liquid layers?
Liquid layers are distinct layers formed by different liquids based on properties like density, temperature, and immiscibility. They often appear in nature, industry, and even household situations.
Why do some liquids form layers?
Liquids form layers primarily due to differences in density and whether or not they can mix (immiscibility). Denser liquids sink, while less dense ones float, creating visible layers.
Can all liquids form layers?
No, only immiscible liquids with different densities form distinct layers. Miscible liquids like alcohol and water mix completely and do not layer.
How do temperature changes affect liquid layers?
Temperature affects density. Warmer liquids are generally less dense and rise, while cooler liquids are denser and sink, contributing to or disrupting existing layers.
Are liquid layers important in nature?
Absolutely. They influence weather, marine ecosystems, and freshwater habitats. They also play a role in heat distribution and nutrient cycling.
Can I create liquid layers at home?
Yes. Using household items like honey, oil, and water, you can create your own liquid layers. It’s a great educational activity.
What industries use liquid layering?
Many industries, including oil refining, food production, pharmaceuticals, and wastewater treatment, rely on understanding and controlling liquid layers.
Do liquid layers stay forever?
No, external factors like movement, temperature changes, or time can disrupt and blend the layers.
What causes immiscibility in liquids?
Molecular structure and polarity determine immiscibility. Polar liquids like water don’t mix with non-polar ones like oil.
Is surface tension important in liquid layers?
Yes, surface tension helps maintain layer boundaries and affects how liquids interact at their interfaces.
Conclusion
Liquid layers may seem like a simple concept, but they offer a window into the complexity of our physical world. From kitchen experiments to global ocean currents, these layers shape ecosystems, influence industry, and fuel scientific discovery. Understanding how and why they form allows us to better navigate, utilize, and protect our environment. Whether you’re a curious student, a seasoned scientist, or just someone fascinated by the little wonders of life, exploring the world of liquid layers is both enlightening and rewarding.